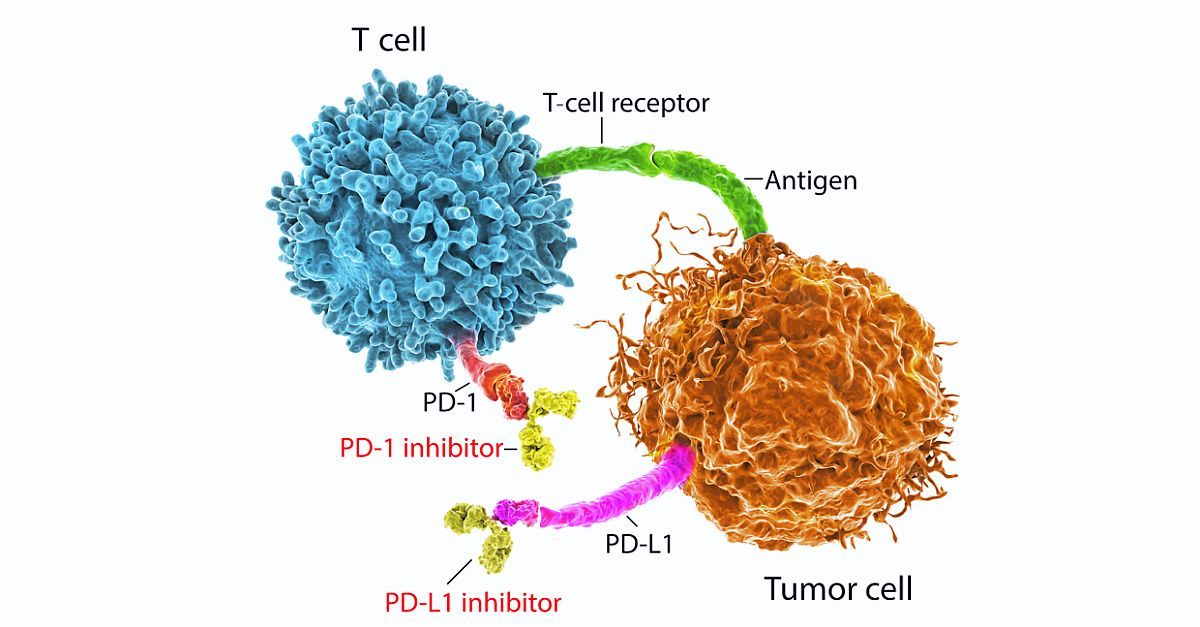
Immune checkpoint inhibitors (ICIs) have had a rapid and broad expansion of their indications in recent months and are now the standard of care for many types of cancer. ICIs promote an antitumor immune response by inhibiting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 /ligand 1 (PD-1/PD-L1), or both. However, this mechanism of action is also associated with immune-related adverse events (irAEs) of varying degrees of severity. Grade 1 irAEs are typically managed with close monitoring, while grade 2 or higher irAEs may require corticosteroids and ICI discontinuation. Some patients with grade 2 or 3 irAEs are able to resume ICI treatment, but current guidelines recommend permanent discontinuation of ICI for grade 4 irAEs.
To characterize the safety of ICI rechallenge after discontinuation due to irAEs, Marion Allouchery and colleagues analyzed data from 180 patients in the French pharmacovigilance database with melanoma, lung cancer, renal cell carcinoma, and other tumor types. The majority (79%) of patients received an anti–PD-1 agent; 10% received an anti–CTLA-4 and anti–PD-1 combination, 6% an anti–CTLA-4 agent, and 5% an anti–PD-L1 agent. Gastrointestinal disorders were the most frequent reason for previous ICI discontinuation, followed by endocrine disorders, hepatitis, respiratory disorders, and skin disorders. Most irAEs leading to discontinuation were grade 2 (52%) or grade 3 (46.5%), with only 1.5% of patients reporting grade 4 irAEs. Median time to rechallenge was 56 days.
There were fewer irAEs following ICI rechallenge; 61% of patients did not experience recurrent grade 2 or higher irAEs after ICI rechallenge. Of the 70 patients (38.9%) who did experience an irAE, most (65%) were grade 2. Grade 3 irAEs were less frequent after rechallenge vs initial treatment, suggesting that ICI rechallenge was not associated with more-severe irAEs. Interestingly, the rates of irAE recurrence were lower among patients rechallenged with the same ICI drug or ICI combination. Also notable, patients with initial gastrointestinal irAEs were more likely to have recurrent irAEs after rechallenge; in contrast, endocrine irAEs were less likely to recur. Median duration from ICI discontinuation to rechallenge and severity of the initial irAE were not predictive of recurrent irAEs after rechallenge. In the majority of patients with an irAE, the second irAE was a recurrence of the first. In about one-quarter of the patients, the second irAE was a new irAE.
Most patients required systemic corticosteroids, and 76.6% of second irAEs resolved to grade 1 or lower. However, 67% of patients with recurrent irAEs discontinued ICI treatment. The authors caution that ICI rechallenge may not be appropriate in patients with a grade 4 irAE or in those who have had neurologic or cardiac irAEs.
High level
The results of this study highlight the value of reporting databases to further characterize the safety profile of ICIs. In this study, a majority of patients who discontinued ICI treatment for grade 2 or higher irAEs received ICI rechallenge with no or few recurrent irAEs. Further prospective studies are needed to help identify risk factors that may influence patient outcomes after ICI rechallenge, inform clinician decision-making, and support future updates to treatment guidelines.
Ground level
In this analysis, ICI rechallenge was not associated with more-severe recurrent irAEs, and appears to be safe under close monitoring, especially in patients who experienced endocrine irAEs with first ICI treatment. However, additional research is needed to further clarify risk factors for recurrent irAEs in patients receiving ICI rechallenge. ICI rechallenge should always be discussed in a multidisciplinary team review of the anticipated benefits, patient comorbidities, and risk of recurrence of first irAEs.