The annual Aptitude Health-TME Take the Lead in Breast Cancer Care Fall Summit was held September 22–23, 2023, in Las Vegas. During the Survivorship section of the program, Dr Anne Peled and participating leaders in breast care discussed the recent inclusion of BIS as an objective screening tool for early detection of breast cancer-related lymphedema. Lymphedema is a devastating side effect that currently impacts 10%–20%1 of patients with breast cancer. Dr Peled and participating specialists highlighted the important addition of objective screening tools, BIS in particular, to detect subclinical lymphedema, before symptoms or swelling appear.
The annual Aptitude Health-TME Take the Lead in Breast Cancer Care Fall Summit was held September 22–23, 2023, in Las Vegas. During the Survivorship section of the program, Dr Anne Peled and participating leaders in breast care discussed the recent inclusion of BIS as an objective screening tool for early detection of breast cancer-related lymphedema. Lymphedema is a devastating side effect that currently impacts 10%–20%1 of patients with breast cancer. Dr Peled and participating specialists highlighted the important addition of objective screening tools, BIS in particular, to detect subclinical lymphedema, before symptoms or swelling appear.
1. Gillespie TC, et al. Gland Surg. 2018;7(4):379-403.
 In routine posttreatment surveillance, regular monitoring for lymphedema using BIS
In routine posttreatment surveillance, regular monitoring for lymphedema using BIS
detects fluid accumulation in the extracellular fluid compartment, before swelling or
changes in standard limb-volume measurements occur. Detecting and eliminating
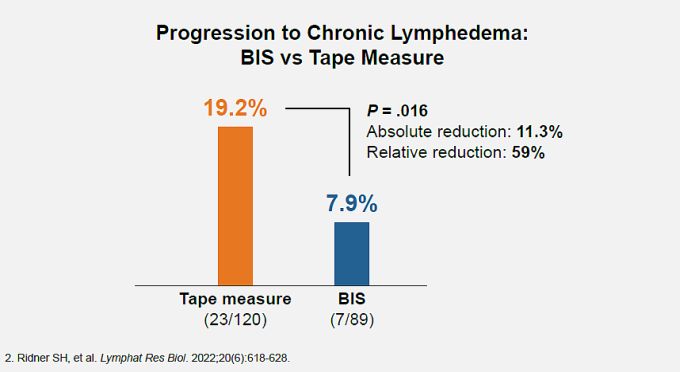
subclinical fluid accumulation (stage 0–1 lymphedema) is the key to preventing progression to irreversible stage 2 and 3 clinical lymphedema, as demonstrated in the PREVENT trial,2 the largest randomized study to date to assess lymphedema prevention. This is because persistent swelling in the extracellular fluid compartment can soon lead to irreversible loss of tissue elasticity, resulting in chronically increased limb volume. PREVENT compared quarterly BIS with rigorous limbvolume measurements, for early detection and prevention of progression to chronic lymphedema through 3 years. In the study, when BIS detected subclinical fluid accumulation, or when tape measurements detected a 5%–10% volume increase, patients were treated with a standard compression garment for 4 weeks. Patients randomized to BIS screening progressed to chronic lymphedema at half the rate seen in patients screened with rigorous tape measurements.
Progression to chronic lymphedema was 59% less likely for patients treated with 1-month compression following a positive BIS screen. (Overall chronic lymphedema rate in the BIS screening group was 70% less than in the tape measure screening group.)
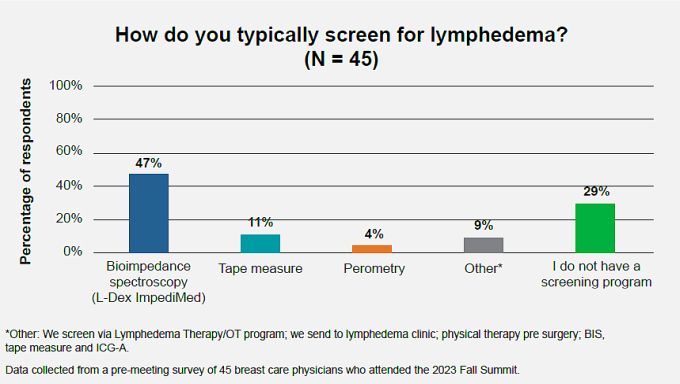
 Including objective BIS measurement in posttreatment surveillance is also in compliance with National Accreditation Program for Breast Centers guidelines for posttreatment lymphedema surveillance. BIS measurements have been recommended in American Physical Therapy Association guidelines for many years. Guidelines organizations also emphasize that obtaining baseline pretreatment measures is ideal to facilitate the most accurate posttreatment monitoring. In our resummit survey of attendees of the Breast Care Fall Summit, we also saw that close to half of attendees already currently use BIS to screen for lymphedema.
Including objective BIS measurement in posttreatment surveillance is also in compliance with National Accreditation Program for Breast Centers guidelines for posttreatment lymphedema surveillance. BIS measurements have been recommended in American Physical Therapy Association guidelines for many years. Guidelines organizations also emphasize that obtaining baseline pretreatment measures is ideal to facilitate the most accurate posttreatment monitoring. In our resummit survey of attendees of the Breast Care Fall Summit, we also saw that close to half of attendees already currently use BIS to screen for lymphedema.
 Currently, ImpediMed’s SOZO® Digital Health Platform with L-Dex is the only
Currently, ImpediMed’s SOZO® Digital Health Platform with L-Dex is the only
US Food and Drug Administration-cleared BIS technology for lymphedema assessment. The TME leadership group suggested that the best placement for the BIS SOZO system is at the check-in station at the surgical or medical clinic point of care. Placement of the BIS SOZO screening encounter outside the standard patient-care workflow (such as solo placement in the physical therapy clinic) leads to underutilization and an inefficient or failed screening program. In keeping with the TME-Aptitude Health mission to foster access to important advances in breast cancer care, we highlight the findings that a lymphedema prevention program that includes objective BIS screening can reduce the chronic lymphedema rate in at-risk patients with breast cancer to around 3%.
To learn more, visit ImpediMed.