Medical Publications
Deliver Trusted Insights
to HCPs
Turn the latest clinical science, health economics and outcomes research (HEOR), and real-world evidence into peer-reviewed literature with our dedicated team of experienced and certified medical publications professionals. Our full range of publication planning and execution services optimizes data dissemination, fulfills educational needs, and addresses barriers to adoption.
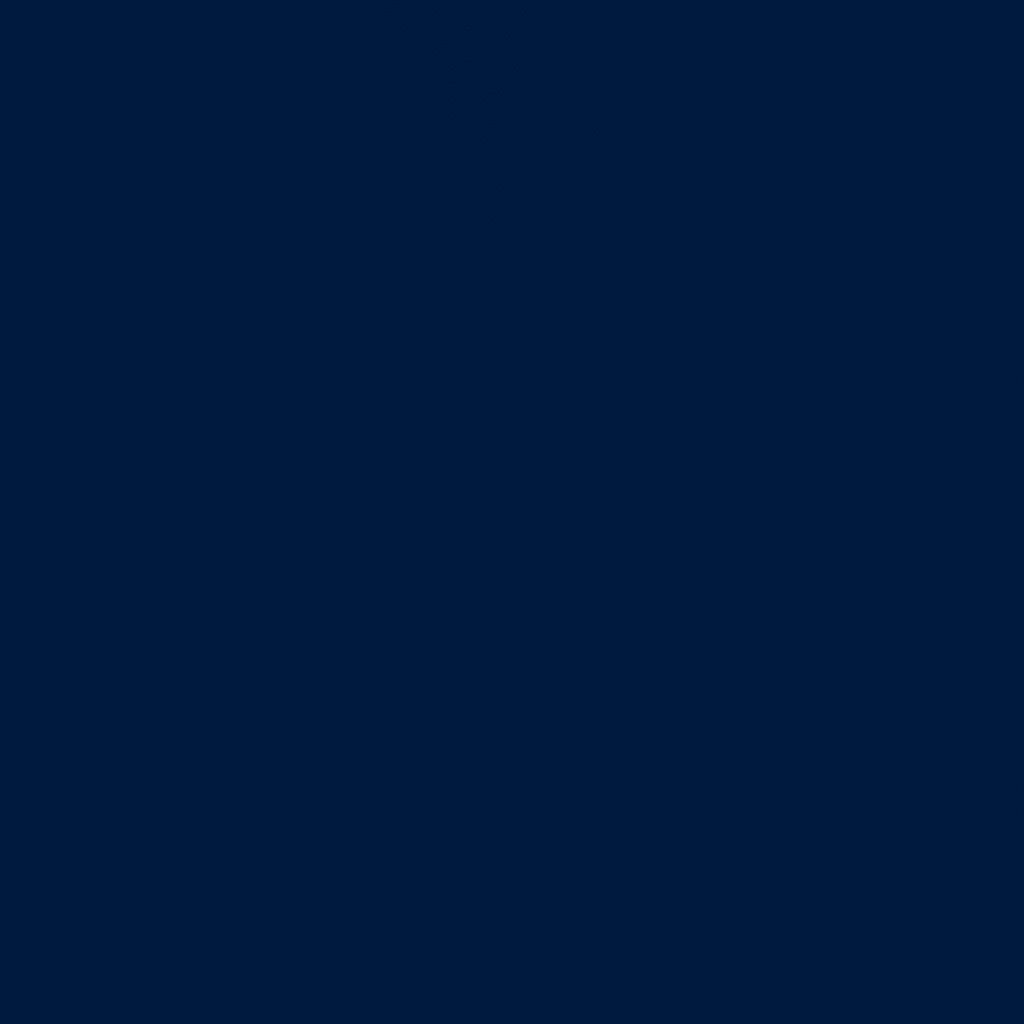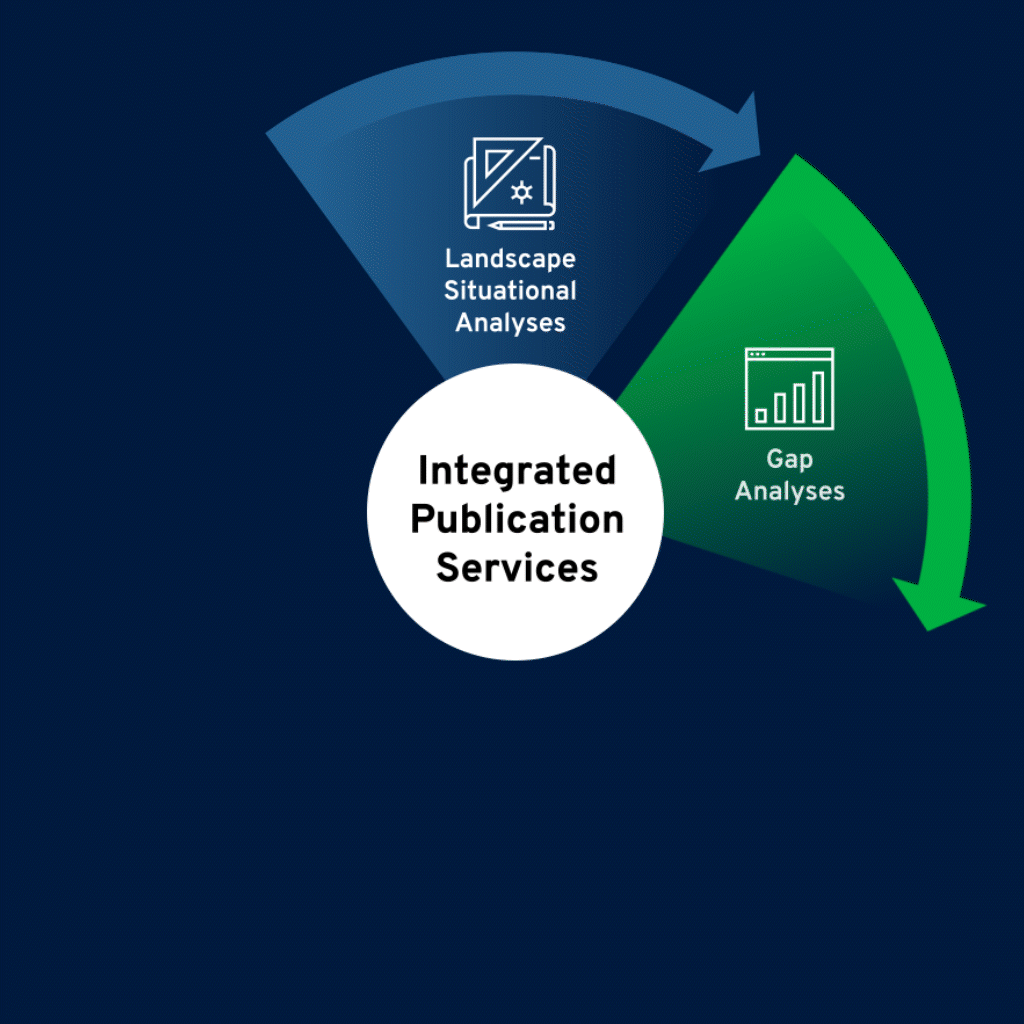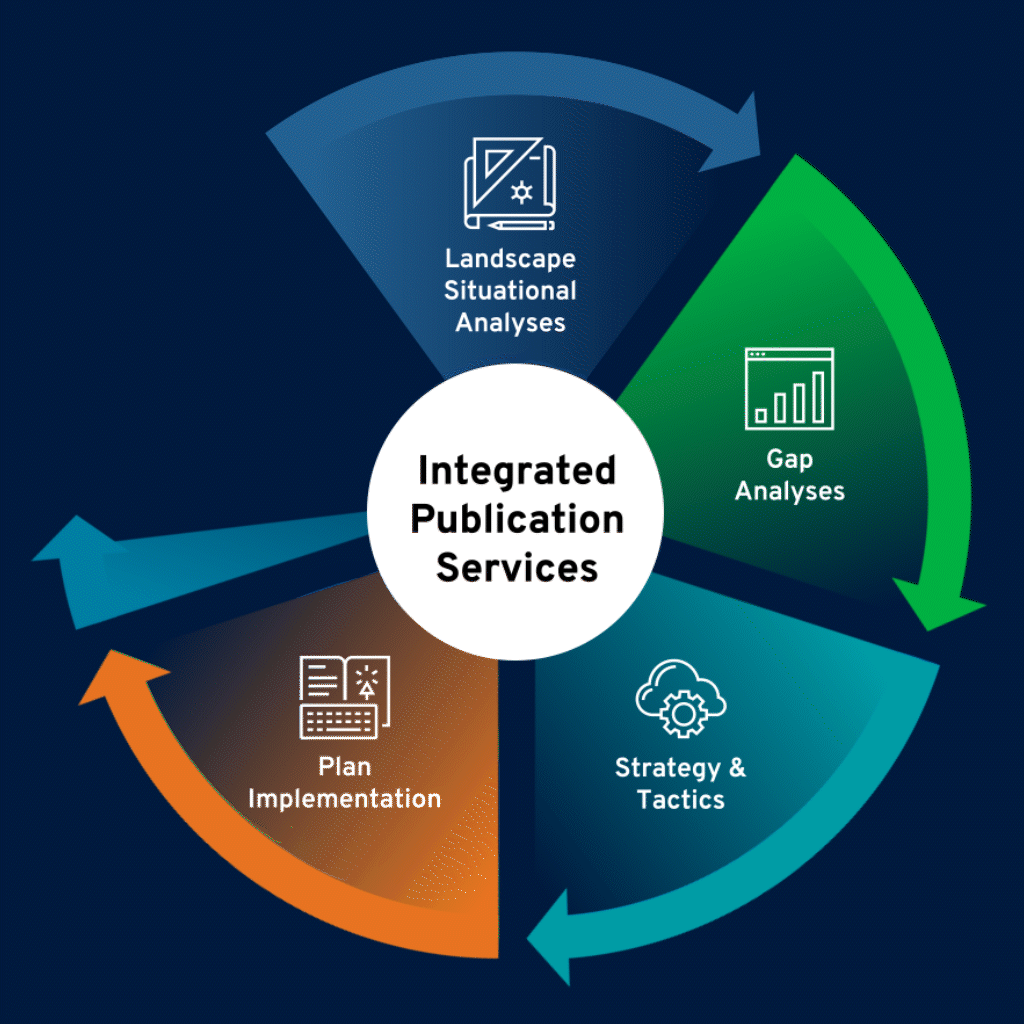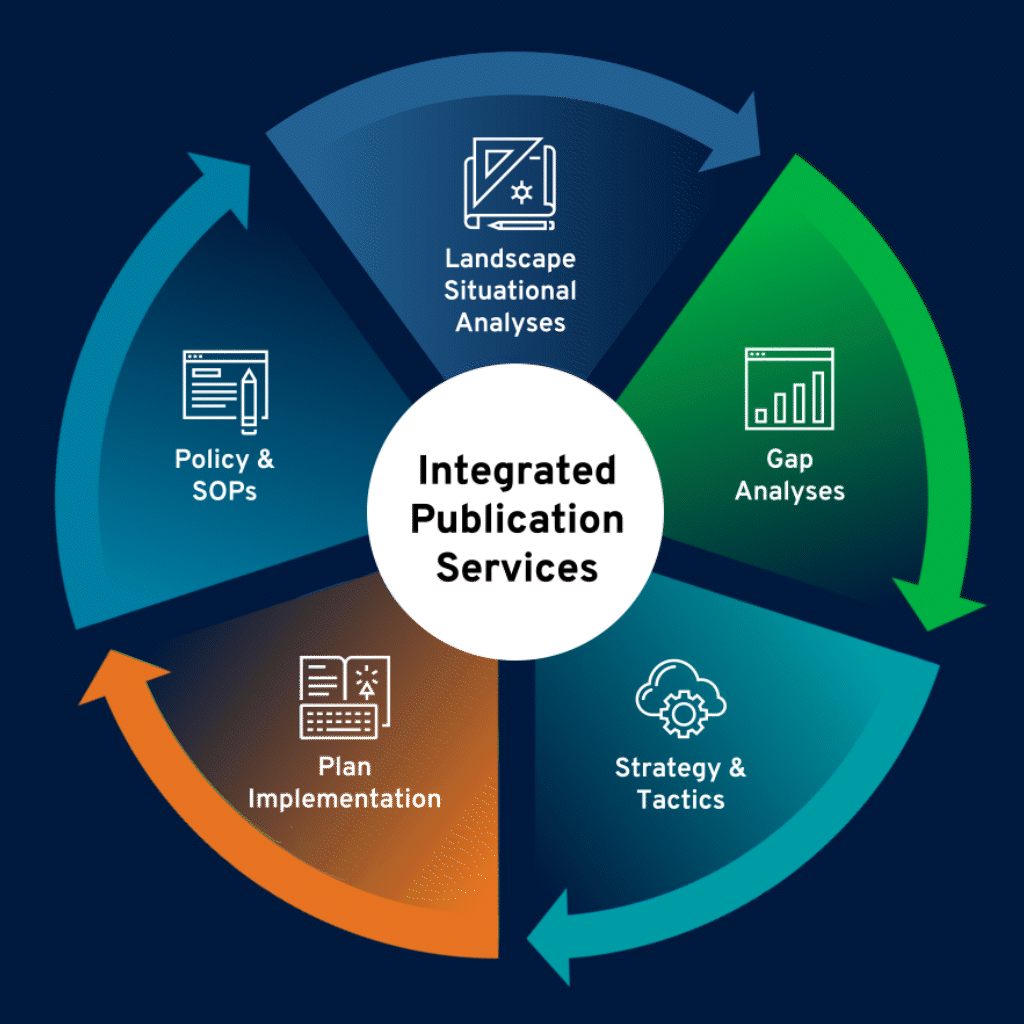
Develop Publication Policy and SOPs
Our team provides robust support for the development of tailored publication policies and standard operating procedures in accordance with Good Publication Practice and the highest industry standards. Our comprehensive services cover the full range of publication policy and SOP planning and execution.
Identify Needs, Challenges, and Opportunities
Thorough landscape situational analyses serve as the foundation for medical publications planning. We conduct a deep dive into specific disease and therapeutic environments, product data, and ongoing trials, as well as competitive landscape.
Assess
Publication Gaps
In-depth gap analyses reveal educational needs and offer insights into a product’s perceptions in the scientific literature. We identify potential gaps in data, share of voice, audiences, and communications, and provide strategic and tactical recommendations to effectively bridge those gaps.
Build a Robust Publication Plan
Our publication plans maximize publication opportunities, ensuring gaps are addressed and relevant audiences are reached. Recommended tactics take client goals and strategic variables into consideration, including overall strategy, educational needs, clinical development programs, and timing of data availability.
Develop Effective Medical Publications Using
Multiple Formats
Our medical publications team facilitates scientific writing and publications management, as well as coordinates communications among stakeholders to ensure timely publications development. We specialize in state-of-the-art, multimedia publications—including print, digital formats (eg, interactive or narrated posters, visual abstracts, author videos and podcasts, infographics)—that are compliant with GPP and journal or congress requirements.
Case Study: Addressing Educational Needs
Learn how our team helped a life science partner increase data dissemination and published manuscripts.
